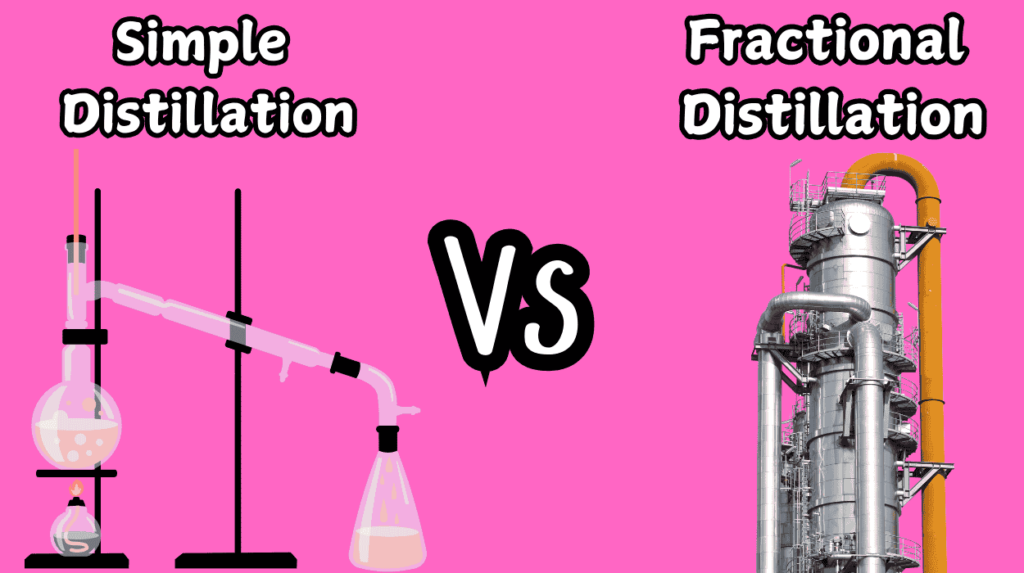
10 Key Difference Between Simple Distillation and Fractional Distillation
As a chemical engineer with hands-on experience in industrial processes, I’ve come to realize how crucial the choice between simple distillation and fractional distillation can be in determining the efficiency, purity, and overall success of a separation. Whether you’re working in a laboratory or dealing with large-scale industrial applications, knowing when and how to apply these techniques can make all the difference.
In this article, we’ll explore the 10 key differences between simple and fractional distillation, covering everything from their setups and energy requirements to their real-world applications in industries like oil refining, pharmaceuticals, and food processing. By the end of this post, you’ll not only have a deeper understanding of these distillation methods but also how they can influence the outcome of a separation process. I’ll also share personal insights and examples drawn from my own experience, as well as key references to enhance your knowledge.
What will you gain?
- A clearer understanding of when to use simple or fractional distillation
- Practical examples of how these techniques are applied in real-world industries
- Insights into the equipment, efficiency, and cost differences
- Tips on how to approach technical interviews related to distillation methods
Let’s dive in and sharpen our understanding of these essential distillation processes! i hope at the end of article youwill get idea about what is the difference between simple distillation and fractional distillation.
Table of Contents
1. Definition1
From my experience, simple distillation is a straightforward process used when the components have significantly different boiling points (typically over 25°C). It’s ideal for separating liquids that are easy to distinguish by boiling point, such as water and ethanol.
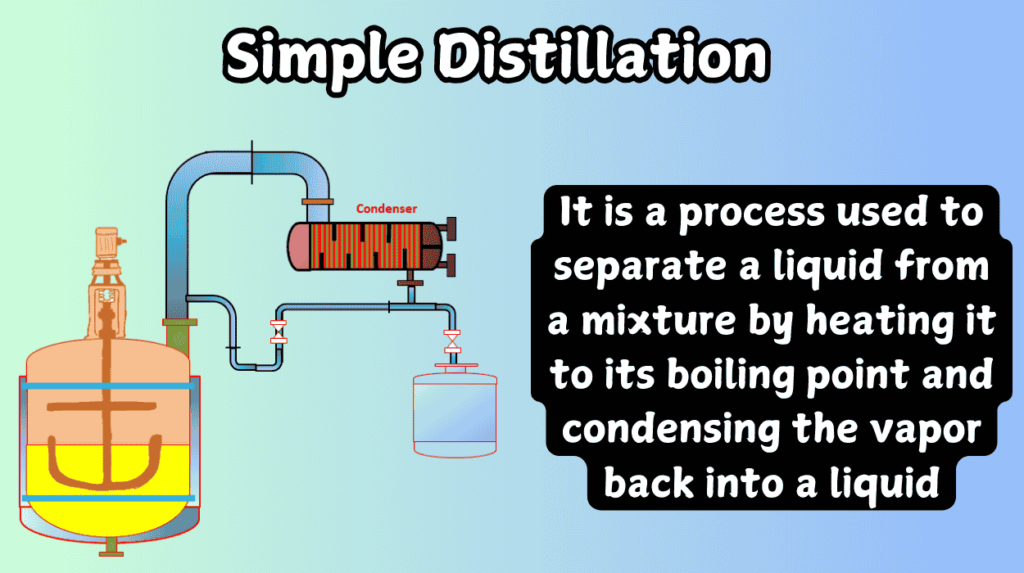
For example, in a laboratory setting, I’ve used simple distillation to separate ethanol from a mixture of water and ethanol. The ethanol boils at around 78°C, while water boils at 100°C. This large difference makes it easy to separate the two using simple distillation.
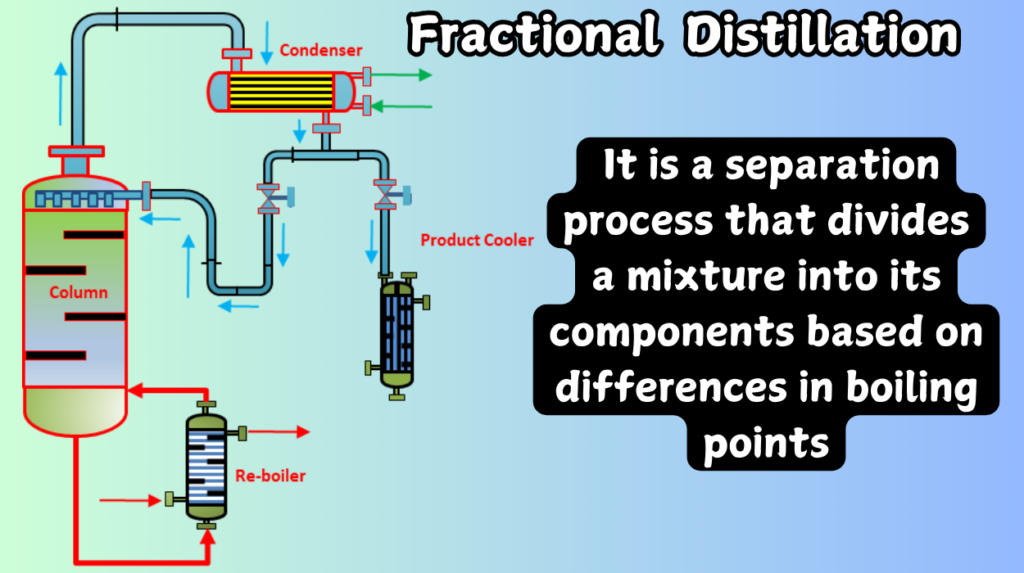
In contrast, fractional distillation is used when the boiling points are close to each other, requiring a more precise separation. A practical example is crude oil refining, where different hydrocarbon fractions (such as gasoline, kerosene, and diesel) have boiling points that differ only slightly, making fractional distillation the method of choice.
2. Setup Complexity2
In my experience, simple distillation3 requires minimal equipment—a distillation flask, a condenser, and a receiver. This simplicity makes it suitable for small-scale applications, this is one of the difference between simple and fractional distillation.
For example, when working in a research laboratory, I’ve set up simple distillation apparatus for solvent recovery, where only a distillation flask, condenser, and receiving flask are needed.
On the other hand, fractional distillation is more complex, involving a fractionating column, which allows the process to separate components more effectively. A fractionating column has multiple trays or packing materials that help in repeated vaporization and condensation cycles, improving the separation efficiency.
In large-scale operations like oil refining, fractional distillation is preferred because it can handle the more complicated separation processes required to separate crude oil into multiple useful components.
3. Efficiency4
I’ve found that fractional distillation is generally more efficient, particularly when separating mixtures with similar boiling points. The fractionating column allows for repeated condensation and vaporization, enhancing the separation process.
For instance, in the petroleum industry, where crude oil is distilled into fractions like gasoline, diesel, and kerosene, fractional distillation proves to be much more efficient than simple distillation because it enables the separation of many components from a complex mixture.
Simple distillation, by contrast, works well when there is a significant difference in boiling points, but it can’t achieve the same level of efficiency for closely boiling components. I’ve used simple distillation to purify water from ethanol, but when trying to separate ethanol from other alcohols or impurities, I needed the precision of fractional distillation, this umber 3 difference between simple and fractional distillation.
4. Number of Components Separated5
Simple distillation is effective when separating just two components, particularly when one has a much lower boiling point than the other. I’ve used it in cases where the goal was to recover a solvent from a solution with little to no complex compounds.
Fractional distillation, on the other hand, is indispensable when dealing with mixtures containing more than two components with similar boiling points. For example, in the distillation of petroleum, fractional distillation allows the separation of a wide range of components into various products, such as gasoline, kerosene, and jet fuel.
In one case, I observed how a single distillation column in a refinery was used to separate crude oil into multiple fractions, showing fractional distillation’s ability to handle complex mixtures, this the number 4 difference between simple and fractional distillation.
5. Energy Requiremen6
Based on my experience, fractional distillation typically requires more energy compared to simple distillation. This is because the fractionating column needs to maintain multiple cycles of heating, condensation, and vaporization. The energy consumption increases with the complexity of the separation, this is one difference between simple and fractional distillation.
For example, when separating various fractions of crude oil, the distillation process requires higher energy inputs to operate the fractionating column, as it’s heating and cooling multiple times. In contrast, simple distillation only requires a single cycle, making it more energy-efficient for simpler separations like removing water from alcohol, this is the number 5 off 10 key differences between simple and fractional distillation.
6. Purity of Output7
In my experience, fractional distillation results in higher purity outputs because of the multiple distillation cycles it performs. For instance, when I was involved in producing high-purity ethanol in the chemical industry, fractional distillation was used to ensure a near-complete separation from other alcohols and impurities, this is one difference between simple and fractional distillation.
For purity types of reflux in distillation column is very important.
Simple distillation, while useful for less complex separations, does not provide the same level of purity. I’ve seen it used in cases where the goal is simply to separate a primary component from a minor impurity, but it wouldn’t be ideal for achieving high-purity separation, this is number 6 of 10 key differences between simple and fractional distillation.
7. Apparatus Cost8
From my perspective, the cost of setting up a fractional distillation9 system is significantly higher due to the need for specialized equipment like the fractionating column. This can be a limiting factor for smaller-scale operations or research projects where the budget is constrained.
In contrast, I’ve used simple distillation setups for smaller-scale operations, such as in laboratory distillation or small-scale solvent recovery, where the equipment cost is minimal. Simple distillation can be much more affordable when only a basic separation is required, this is number 7 10 key differences between simple and fractional distillation, this is one difference between simple and fractional distillation.
8. Industrial Applications10
Simple distillation is more commonly used in small-scale applications, such as laboratory settings or small solvent recovery units. I’ve worked on projects where simple distillation was ideal for separating a single liquid from a solution.
In contrast, fractional distillation is widely used in large-scale operations, such as crude oil refining, where it is essential to separate multiple components from complex mixtures. For example, when I worked on a project involving the separation of different alcohols and esters, fractional distillation was necessary for handling the complexity of the mixture.
9. Scale of Operation11
In my time working with various distillation processes, I’ve seen that simple distillation is better suited for small-scale operations. For example, in a small pilot plant or laboratory, simple distillation is often employed for solvent purification.
For larger-scale operations, such as in the petrochemical industry, fractional distillation is a must. The process can handle the volume of feedstock required for industrial operations. I’ve seen fractional distillation applied in large refineries to separate crude oil into multiple valuable products, this one difference between simple and fractional distillation.
10. Time Required12
In my experience, simple distillation is faster since it only requires one cycle of vaporization and condensation. For instance, I’ve used simple distillation to separate solvents in a quick turnaround, especially when only one primary component is involved.
Read Article on Line sizing calculation with example
However, fractional distillation takes more time due to the continuous cycles within the fractionating column. In oil refining, for example, the distillation of crude oil can take hours to separate the different fractions properly, but the time spent is well worth the quality and precision of the separation.
10 Questions and Answers About Simple and Fractional Distillation (Interview on Distillation Processes)
1. What is the main difference between simple and fractional distillation?
Simple distillation is used for separating liquids with significantly different boiling points, while fractional distillation is used for liquids with close boiling points.
2. Why is fractional distillation more efficient than simple distillation?
Fractional distillation is more efficient because it uses a fractionating column, which allows repeated cycles of vaporization and condensation to separate mixtures with similar boiling points.
3. What equipment does fractional distillation require that simple distillation does not?
Fractional distillation requires a fractionating column, which is necessary for effective separation of liquids with similar boiling points.
4. How does the time required differ between the two distillation methods?
Simple distillation is faster because it involves only one vaporization-condensation cycle, while fractional distillation involves multiple cycles in the fractionating column.
5. How does the purity of the output compare in both methods?
Fractional distillation yields a purer distillate because of the continuous separation in the fractionating column, while simple distillation may leave impurities when separating liquids with close boiling points.
6. How do the costs of the setups differ?
Fractional distillation setups are more costly due to the addition of the fractionating column and associated components, whereas simple distillation setups are relatively low-cost.
7. In what industries is each method most commonly used?
Simple distillation is commonly used in laboratory settings and small-scale alcohol distillation, while fractional distillation is used extensively in the petroleum, chemical, and pharmaceutical industries.
8. How does the scale of operation influence the choice of distillation method?
Simple distillation is ideal for small-scale operations, while fractional distillation is suitable for both small and large-scale industrial applications, especially when complex separations are required, difference between simple and fractional distillation.
9. Which method requires more energy?
Fractional distillation generally requires more energy because of the multiple condensation-evaporation cycles within the fractionating column.
10. Why is fractional distillation preferred for complex mixtures?
Fractional distillation is preferred for separating complex mixtures with components that have similar boiling points, as the fractionating column enhances separation efficiency through multiple cycles of vaporization and condensation.
Pro Tips for Interview Preparation
If you’re preparing for an interview related to distillation processes or seeking to gain a deeper understanding of distillation techniques, here are some pro tips based on my personal experience:
- Understand the Fundamentals: Make sure you understand the basic principles of both simple and fractional distillation. Know when to use each technique, and be ready to explain why one is more suitable than the other for specific applications.
- Learn About Industrial Applications: Familiarize yourself with real-world examples where these distillation processes are applied, such as crude oil refining or solvent recovery. Knowing these examples will help you connect theoretical concepts to practical scenarios, difference between simple and fractional distillation.
- Know the Equipment: Be prepared to discuss the equipment used in both processes, including the fractionating column for fractional distillation and the setup for simple distillation. Understand the differences in their construction and operation.
- Energy and Efficiency Considerations: Be ready to discuss the energy requirements for both distillation methods, and explain why fractional distillation typically consumes more energy than simple distillation.
- Purity and Yield: Understand how purity and yield are affected by the choice of distillation process. For example, be able to explain why fractional distillation produces higher purity products.
- Stay Updated on Current Trends: Research any advancements in distillation technology, such as new types of fractionating columns or improvements in energy efficiency.
- Ask Questions: Interviews are a two-way street. Don’t hesitate to ask about the company’s applications of distillation processes or the specific challenges they face in separating complex mixtures.
- Review Key Textbooks: Reviewing key textbooks like Perry’s Chemical Engineers’ Handbook and Unit Operations of Chemical Engineering will solidify your theoretical knowledge and give you a solid foundation for answering technical questions.
Conclusion
Preparing for an interview on distillation processes requires both a solid understanding of theoretical concepts and practical experience. Whether you are tackling questions about equipment design, process optimization, safety protocols, or real-world applications, having a well-rounded approach will help you stand out, it also covers interview on distillation processes.
I hope the insights shared in this article have been helpful as you get ready for your next interview. Remember, each interview is a learning opportunity, and being prepared is the key to success.
I’d love to hear your thoughts! Do you have any experiences or tips of your own when it comes to preparing for technical interviews? Please feel free to share your feedback in the comments below. Also, if you found this article useful, don’t forget to subscribe for more helpful guides, tips, and resources on engineering, process design, and other industry insights.
Stay prepared, stay confident, and best of luck with your interviews!
References
- McCabe, W. L., Smith, J. C., & Harriott, P. (2005). Unit Operations of Chemical Engineering (7th Edition). McGraw-Hill ↩︎
- Perry, R. H., Green, D. W., & Maloney, J. O. (2008). Perry’s Chemical Engineers’ Handbook (8th Edition). McGraw-Hill. ↩︎
- Fogler, H. S. (2006). Elements of Chemical Reaction Engineering (4th Edition). Prentice Hall. ↩︎
- Dunn, R. M., & Araujo, R. (1995). Distillation Design. John Wiley & Sons. ↩︎
- King, C. J. (1980). Separation Processes. McGraw-Hill. ↩︎
- Seader, J. D., & Henley, E. J. (2006). Separation Process Principles. Wiley. ↩︎
- Davis, R. (2009). Introduction to Chemical Engineering: Tools for Today and Tomorrow. McGraw-Hill. ↩︎
- Sorrell, S. (2007). The Chemistry of Petroleum. CRC Press. ↩︎
- Stanley, P. R., & Sander, S. (2004). Chemical Engineering Design: Principles, Practice, and Economics of Plant and Process Design. Elsevier ↩︎
- Wankat, P. C. (2007). Rate-Controlled Separations. Elsevier. ↩︎
- Simple Distillation Wikipedia ↩︎
- Fractional Distillation Wikipedia ↩︎
Google Search
What is the difference between simple distillation and fractional distillation
Discover more from Technical Guide
Subscribe to get the latest posts sent to your email.

1 thought on “10 Key Difference Between Simple Distillation and Fractional Distillation”
Comments are closed.